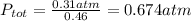Answer:
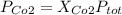
Where
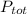 represent the total pressure and
represent the total pressure and
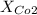 the fraction of carbon dioxide is 0.46 and we can find the total pressure with this formula:
the fraction of carbon dioxide is 0.46 and we can find the total pressure with this formula:
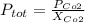
And replacing we got:
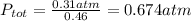
Explanation:
For this case the partial presure of carbon dioxide is given by:
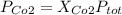
Where
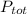 represent the total pressure and
represent the total pressure and
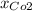 the fraction of carbon dioxide is 0.46 and we can find the total pressure with this formula:
the fraction of carbon dioxide is 0.46 and we can find the total pressure with this formula:
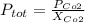
And replacing we got: