Answer:
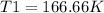
Step-by-step explanation:
According to Gay-Lussac’s law simply states that the ratio of the initial pressure and temperature is equal to the ratio of the final pressure and temperature for a gas of a fixed provided that the mass is kept at a constant volume.
Given:
Initial pressure, P1 = 1 atm
Final pressure, P2 = 1.5 atm
Final temperature, T2 = 250 K
The law can be applied using the below formula
P1T2 = P2T1
Then,
T1 = (P1T2)/P1 = (1*250)/(1.5) = 166.66 Kelvin.
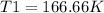
: