Answer:
0.18 Moles
Step-by-step explanation:
first you need to convert the 5.4g to moles using molar mass
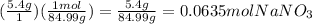 )
)
once you have that done you can use the molarity equation to get the answer
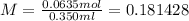
now you need to round to the correct sig fig which will give you the correct answer of 0.18moles