Answer:
A saturated solution with excess sodium chloride undissolved
Step-by-step explanation:
Hello,
In this case, since 32.6 g of sodium chloride are completely dissolved in 100 g of water at 25 °C, we can propose a rule of three to compute the grams of sodium chloride which are completely dissolved but in 75.0 g of water as shown below:
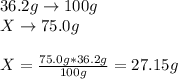
For that reason, since 29.5 g of sodium chloride are attempted to be dissolved, the following amount will remain undissolved:
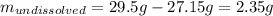
Thus, since the undissolved amount is low we can conclude it is a saturated solution with excess sodium chloride undissolved since 2.35 g will remain undissolved.
Best regards.