Answer:
1000 K
Step-by-step explanation:
Given data
- Moles of carbon dioxide (n): 0.5 moles
- Volume of carbon dioxide (V): 50 liters
- Pressure of carbon dioxide (P): 0.8210 atmospheres
- Temperature of carbon dioxide (T): ?
We can find the temperature of carbon dioxide using the ideal gas equation.
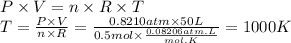
The temperature of the gas is 1000 K.