Answer:
1.505*10^{24} molecules
Step-by-step explanation:
The number of molecules is independent of the subtance, and only depends on the number of moles.
To calculate the number of molecules in a substance with n moles you use the following formula:
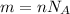
m: number of molecules
NA: Avogadro's number = 6.02*10^23 molecules/mol
n: moles of the substance = 2.5 mol
You replace the values of n and NA in the equation for m and you obtain:
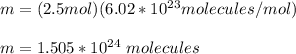
hence, the number of molecules is 1.505*10^24