Answer:
0.57 mol/L
Step-by-step explanation:
Molarity (M) is defined as the number of moles of solute per liter of solution (moles/Liter).
Molarity =
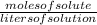 or Moles = molarity × litres
or Moles = molarity × litres
so, the mass of Nacl in 1 mol is 58.44 g,
Moles of NaCl = 50.0g x 1 mole NaCl / 58.44 g
= 0.855 mol NaCl
Moles = molarity × litres
0.855 = 1.5 M x L
L = 0.57 mol/L
Hence, 0.57 mol/L can be prepared from 50.0 g of NaCl.