Answer:
The equilibrium concentration of gas A = 1.43 M
Step-by-step explanation:
First set up the equilibrium expression:
![K = ([C]* [D])/([A]* [B])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/vlmzzrrmogmxyfcjk9ddu2z0riq8uqmq7s.png)
The problem states that each product has a concentration of 2.3M at equilibrium, and that k=2.6
We also know that [A] and [B] are the same.
Since we are solving for [A] I'm going to change [A][B] to A²
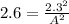
Now we just need to solve for A²
A² = 5.29 / 2.6
A² = 2.0346
A = √2.0346
A = 1.43
Therefore, the equilibrium concentration of gas A = 1.43 M.