Complete Question
Consider the total ionic equation below.

Which is the net ionic equation for the reaction?
A

B
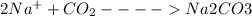
C
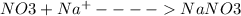
D

Answer:
Option A is the correct option
Step-by-step explanation:
The given ionic equation is

Looking at this complete ionic equation we see that
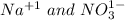 did not change in the reaction so they will not be included in the net ionic equation so the net ionic equation becomes
did not change in the reaction so they will not be included in the net ionic equation so the net ionic equation becomes
