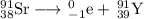Answer:
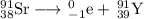
Step-by-step explanation:
The unbalanced nuclear equation is
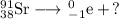
Let's write the question mark as a nuclear symbol.
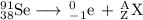
The main point to remember in balancing nuclear equations is that the sums of the superscripts and the subscripts must be the same on each side of the equation.
Then
98 = 0 + A, so A = 98 - 0 = 98, and
38 = -1 + Z, so Z = 38 + 1 = 39
Then, your nuclear equation becomes
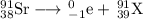
Element 39 is yttrium, so the balanced nuclear equation is