Answer:
P IBr: 15.454atm
I₂: 0.923 atm
P Br₂: 0.923atm
Step-by-step explanation:
Basados en la reacción:
I₂(g) + Br₂(g) ⇄ 2 IBr(g)
La constante de equilibrio, Keq, es definida como:
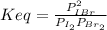
Se cumple la relación de Keq = 280 cuando las presiones están en equilibrio
Usando PV = nRT, la presión inicial de IBr es:
P = nRT / V; 0.500mol*0.082atmL/molK*423.15K / 1.00L = 17.3 atm
Siendo las presiones en equilibrio:
P IBr: 17.3 - 2X
P I₂: X
P Br₂: X
Donde X representa el avance de reacción.
Remplazando en Keq:
280 = (17.3 - 2X)² / X²
280X² = 4X² - 69.2X + 299.29
0 = -276X² - 69.2X + 299.29
Resolviendo para X:
X = -1.174 → Solución falsa. No existen presiones negativas
X = 0.923 → Solución real
Así, las presiones parciales en equilibrio de cada compuesto son:
P IBr: 17.3 - 2X = 15.454atm
P I₂: X = 0.923atm
P Br₂: X = 0.923atm