Answer:
D) 7.5 grams
Step-by-step explanation:
Hello,
In this case, since the percent yield is defined by:
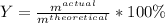
In such a way, since the percent yield is up to 25% and the expected or theoretical amount is 30 g of the product, the actual yield of the reaction is:
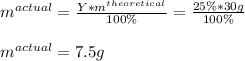
Therefore the answer is D) 7.5 grams.
Best regards.