Answer:
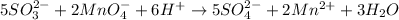
Step-by-step explanation:
Hello,
In this case, given the reaction:
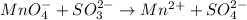
We first identify the oxidation state of both manganese and sulfur at each side:
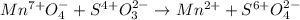
So we have the oxidation and reduction half-reactions below, including the addition of water and hydronium as it is in acidic media:
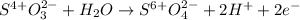
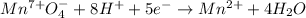
Next, we exchange the transferred electrons:
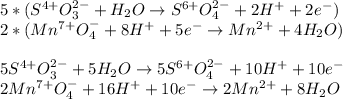
Then we add the resulting half-reactions and simplify the transferred electrons:
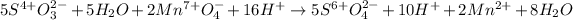
We rearrange the terms in order to simplify water and hydronium molecules:
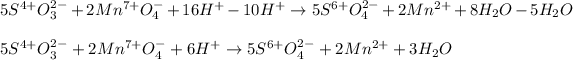
Finally we write the balanced reaction in acidic media:
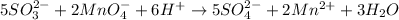
Best regards.