Answer:
The combustion of 59.7 grams of methane releases 3320.81 kilojoules of energy
Step-by-step explanation:
Given;
CH₄ + 2O₂ → CO₂ + 2H₂O, ΔH = -890 kJ/mol
From the combustion reaction above, it can be observed that;
1 mole of methane (CH₄) released 890 kilojoules of energy.
Now, we convert 59.7 grams of methane to moles
CH₄ = 12 + (1x4) = 16 g/mol
59.7 g of CH₄
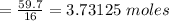
1 mole of methane (CH₄) released 890 kilojoules of energy
3.73125 moles of methane (CH₄) will release ?
= 3.73125 moles x -890 kJ/mol
= -3320.81 kJ
Therefore, the combustion of 59.7 grams of methane releases 3320.81 kilojoules of energy