Answer:
1. Increase volume.
2. No change.
3. No change.
Step-by-step explanation:
Hello,
In this case, if we want to shift the reaction rightwards, based on the Le Chatelier's principle we would have to:
1. For this reaction:
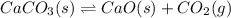
- Increase the volume or decrease the pressure, since there are more gaseous moles at the products.
2. For this reaction:
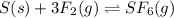
- Do nothing since it is not possible to achieve it as we have the same number of gaseous moles at both reactants and products.
3. For this reaction:
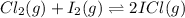
- Do nothing since it is not possible to achieve it as we have the same number of gaseous moles at both reactants and products.
Regards.