Answer:
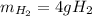
Step-by-step explanation:
Hello,
In this case, water decomposition is represented by:
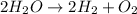
Thus, since water molar mass is 18 g/mol and hydrogen molar mass is 2 g/mol and they are in a 2:2 molar ratio, the produced grams of hydrogen result:
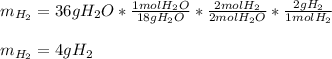
Best regards.