Answer:
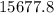 grams of HCl is required to produce 215 moles of lead (II) chloride
grams of HCl is required to produce 215 moles of lead (II) chloride
Step-by-step explanation:
The balanced equation for this reaction is
2HCl + PbO -----> PbCl2 + H2O
2 molecule of HCl combines with one molecule of PbO to form one molecule of PbCl2 and one molecule of H2O
Molar mass of lead (ii chloride) -
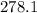 g/mol
g/mol
Mass of
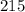 moles of lead (II) chloride is
moles of lead (II) chloride is
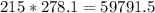
Mass of two molecules of HCl
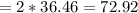 grams
grams
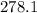 gram of PbO2 is formed by
gram of PbO2 is formed by
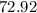 grams of HCL
grams of HCL
1 gram of PbO2 is formed by
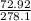 grams of HCL
grams of HCL
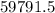 gram of PbO2 is formed by
gram of PbO2 is formed by
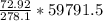 grams of HCL
grams of HCL
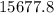 grams of HCl is required to produce 215 moles of lead (II) chloride
grams of HCl is required to produce 215 moles of lead (II) chloride