Answer:
The correct answer to the following question will be Option C (-10 J).
Step-by-step explanation:
The given values are:
Work done by the system, W = 40 J
Thermal energy, Q = 30 J
Change in internal energy, U = ?
As we know,
⇒
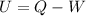
On putting the values in the above expression, we get
⇒
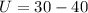
⇒
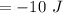
So that the change in internal energy will be "-10 J".