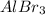Answer:
0.088 mole
Step-by-step explanation:
0.088 mole of Al would be necessary.
From the balanced equation of reaction:
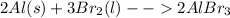
The mole ratio of Al to
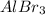 is 1:1, meaning that 1 mole of the former is required to produce 1 mole of the latter.
is 1:1, meaning that 1 mole of the former is required to produce 1 mole of the latter.
Recall that: mole = mass/molar mass
Molar mass of
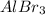 = 266.7 g/mol
= 266.7 g/mol
23.6 g of
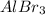 = 23.6/266.7 = 0.088 mole
= 23.6/266.7 = 0.088 mole
Since the mole ratio of the two compounds according to the balanced equation of reaction is 1:1, it thus means that 0.088 moles of Al will also be needed for the reaction.
Hence, 0.088 mole of Al are necessary to produce 23.6 g of