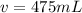Answer:
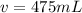 of HCl
of HCl
Step-by-step explanation:
Hcl + KOH. -------> KCl + H2O
the number of moles of KOH. can be calculated by converting the mass to molar mass, then, the mass is divided by it's molecular weight I.e
n = m/M
n = 6.00g/56.106g/mol
n = 0.106940434 mol
n= 0.107 mols of HCl
To calculate the Concentration
C = n/v
where n = number of moles and v = volume
0.225M = (0.106940434 mol)/v
v = (0.106940434 mol)/(0.225M)
v = 0.475290818 L × 1000mL HCl