Answer:
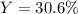
Step-by-step explanation:
Hello,
In this case, given the reaction, the molar mass of ethene is 28 g/mol and the molar mass of carbon dioxide is 44 g/mol. With that information we compute the theoretical yield considering a 1:2 molar ratio respectively between them:
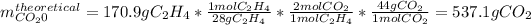
Thus, we compute the percent yield with the given grams of carbon dioxide:
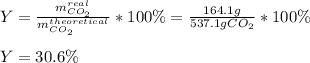
Regards.