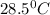Answer: The final temperature of the mixture will be
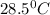
Step-by-step explanation:
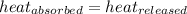
As we know that,
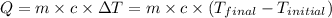
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2021/formulas/chemistry/college/8mq914tycmkoswztk5p5my04anxw73dmzg.png) .................(1)
.................(1)
where,
q = heat absorbed or released
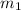 = mass of mercury = 425 g
= mass of mercury = 425 g
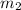 = mass of water = 145 g
= mass of water = 145 g
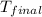 = final temperature = ?
= final temperature = ?
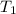 = temperature of mercury =
= temperature of mercury =
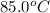
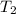 = temperature of water =
= temperature of water =
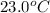
 = specific heat of mercury =
= specific heat of mercury =
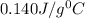
 = specific heat of water=
= specific heat of water=
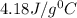
Now put all the given values in equation (1), we get
![-425* 0.140* (T_(final)-85.0)=[145* 4.184* (T_(final)-23.0)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/pxf3d7jh775alhqhf82q0fii3cw541xmkm.png)

Therefore, the final temperature of the mixture will be