Answer: the pH moves to 0 on the pH scale
Step-by-step explanation:
A base is defined as a substance which donates hydroxide ions when dissolved in water and an acid is defined as a substance which donates hydrogen ions in water.
Neutralisation reaction is defined as the chemical reaction in which an acid reacts with a base to produce a salt and water molecule. The
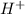 ions from acid are neutralised by
ions from acid are neutralised by
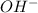 ions from base.
ions from base.
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH of 7.