Answer:
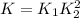
Step-by-step explanation:
Hello,
In this case, the first two steps are:
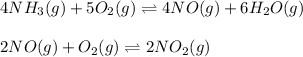
And the net reaction:
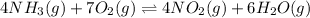
Thus, each equilibrium constant turn out:
![K_1=([NO]^4[H_2O]^6)/([NH_3]^4[O_2]^5)\\ \\K_2=([NO_2]^2)/([NO]^2[O_2])](https://img.qammunity.org/2021/formulas/chemistry/college/bqx2vgeartvt4qishtimp6az6ild28tslp.png)
Thus, if we want to obtain the net equation, we must double the second reaction as:
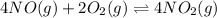
Thereby, its equilibrium constant changes:
![K_2^(new)=([NO_2]^4)/([NO]^4[O_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/5pkycxge9zis1k9lzmdga3oz1zdt08j1o6.png)
Becoming a squared version of the original K₂. In such a way, if we multiple the two equilibrium constants:
![K_1*K_2^2=([NO]^4[H_2O]^6)/([NH_3]^4[O_2]^5)*([NO_2]^4)/([NO]^4[O_2]^2)=([H_2O]^6[NO_2]^4)/([NH_3]^4[O_2]^7)](https://img.qammunity.org/2021/formulas/chemistry/college/qyr0kved5nk1f6o6ij89eh1lbmyuz7hsuq.png)
Next, we express the net reaction equilibrium constant, that is:
![K=([H_2O]^6[NO_2]^4)/([NH_3]^4[O_2]^7)](https://img.qammunity.org/2021/formulas/chemistry/college/9i7xdeyz9yoqbxchqpsqmkmvjhd3vkb525.png)
Therefore, K in terms of K₁ and K₂ results:
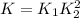
Best regards.