Answer:
Reaction proceeds to the left toward the reactants.
Step-by-step explanation:
Hello,
In this case, since the reaction is:
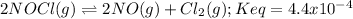
For the given amounts, one computes the reaction quotient:
![Q=([NO]^2[Cl_2])/([NOCl]^2) =((0.500M)^2(0.500M))/((1.00M)^2) =0.125](https://img.qammunity.org/2021/formulas/chemistry/college/53apl0uhouwnt5qlop6z8tugyorjjulunt.png)
Thus, since Q>Keq, the reaction will form more NOCl in order to increase its concentration and therefore reestablish the equilibrium, for that reason the answer is reaction proceeds to the left toward the reactants.
Regards.