Answer:
a.
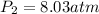
b.
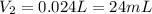
Step-by-step explanation:
Hello,
a. In this case, we use the Boyle's law as en inversely proportional relationship between pressure and volume:

Thus, for the given conditions, one computes the new pressure as shown below:
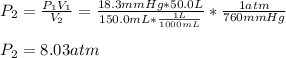
b. Now, we should find the final volume for a new pressure of 10 atm:
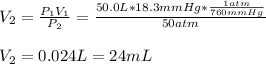
Best regards.