Answer:
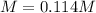
Step-by-step explanation:
Hello,
In this case, we define the molarity as the ratio between the moles of the solute, NaOH here, and the volume of the solution in litres:
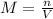
For that reason, one must first compute the moles of sodium hydroxide by using its molar mass of 40 g/mol:
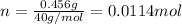
Then, the volume in litres:
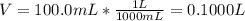
Finally we compute the molarity:
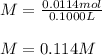
Best regards.