Answer:
the atomic packing factor of Sn is 0.24
Step-by-step explanation:
a = b = 5.83A and c = 3.18A.
Volume of unit cell = a²c
= (5.83)² * 3.18 * 10⁻²⁴ cm³
= 1.08 * 10⁻²²cm³
Volume of atoms =
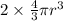
(∴ BCC, effective number of atom is 2)
Volume of atoms =
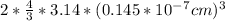
= 2.55*10⁻²³cm³
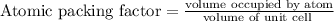
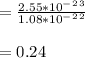
therefore, the atomic packing factor of Sn is 0.24