Answer:
E_T = 36.95J
6. 35.391918182373
Step-by-step explanation:
To find the thermal energy of the total amount of molecules you use the following formula:
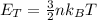 ( 1 )
( 1 )
n: number of molecules = 6.07*10^21
T: temperature = 21°C = 294.15K
k_B: Boltzman's constant = 1.38*10^-23 J/K
You replace the values of the parameters in the equation (1):
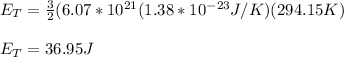
hence, the thermal energy is 36.95J
6. 35.391918182373