Answer:
D. n=6 to n=2
Step-by-step explanation:
Given;
energy of emitted photon, E = 3.02 electron volts
The energy levels of a Hydrogen atom is given as; E = -E₀ /n²
where;
E₀ is the energy level of an electron in ground state = -13.6 eV
n is the energy level
From the equation above make n, the subject of the formula;
n² = -E₀ / E
n² = 13.6 eV / 3.02 eV
n² = 4.5
n = √4.5
n = 2
When electron moves from higher energy level to a lower energy level it emits photons;
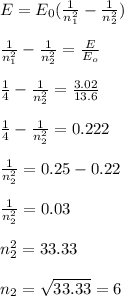
For a photon to be emitted, electron must move from higher energy level to a lower energy level. The higher energy level is 6 while the lower energy level is 2
Therefore, The electron energy-level transition is from n = 6 to n = 2