Answer:
The volume of the neon gas is 17.07 L.
Step-by-step explanation:
The ideal gas equation describes the relationship among the four variables P, V, T, and n. An ideal gas is a hypothetical gas whose pressure-volume-temperature behavior can be completely accounted for by the ideal gas equation.
In order to calculate the volume, first we need to convert the grams of neon to moles:
28.18 g Ne ------ 1 mol Ne
5.0 g Ne---------- x= 0.25 mol Ne
Now, using the ideal gas equation we calculate the volume:
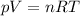
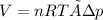
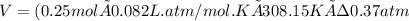
V= 17.07 L