Answer: When the reaction reaches equilibrium, the cell potential will be 0.00 V
Step-by-step explanation:
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
The equilibrium is dynamic in nature and the reactions are continuous in nature. Rate of forward reaction is equal to the rate of backward reaction.
The standard emf of a cell is related to Gibbs free energy by following relation:

The Gibbs free energy is related to equilibrium constant by following relation:
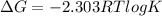
For equilibrium
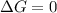
Thus
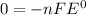
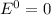
Thus When the reaction reaches equilibrium, the cell potential will be 0.00 V