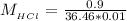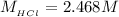Answer:
1
The heat of reaction is
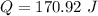
The enthalpy is
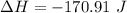
2
The concentration of HCl is
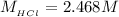
Step-by-step explanation:
From the question we are told that
The volume of
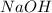 is
is
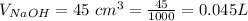
The number of concentration of
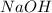 is
is
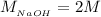
The volume of HCl is
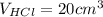
The number of concentration of
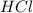 is
is
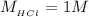
The temperature difference is
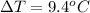
Now the heat of reaction is mathematically represented as
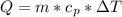
Where
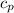 is the specific heat of water with value
is the specific heat of water with value
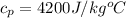
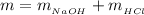
Now
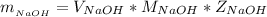
where
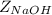 is the molar mass of NaOH with the value of 0.04 kg/mol
is the molar mass of NaOH with the value of 0.04 kg/mol
So
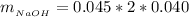
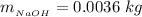
While
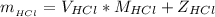
Where
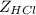 is the molar mass of
is the molar mass of
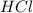 with the value of 0.03646 kg/mol
with the value of 0.03646 kg/mol
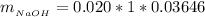
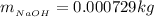
So
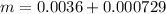
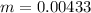
=>
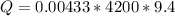
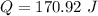
The enthalpy is mathematically represented as
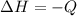
=>
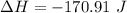
From the second question we are told that
The volume of HCl is
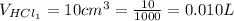
The volume of NaOH is
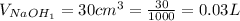
The concentration of NaOH is
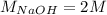
The first temperature change is
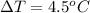
The second volume of
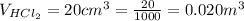
The mass of NaOH is
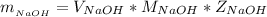
substituting values
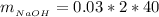
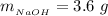
The mass of the product formed is
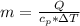
substituting values
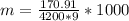
The multiplication by 1000 is to convert it from kg to grams
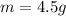
Now the mass of HCl is
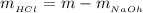
substituting values
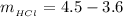
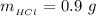
Now the concentration of HCl is
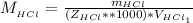
The multiplication of
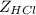 is to convert it from kg/mol to g/mol
is to convert it from kg/mol to g/mol