Answer:
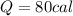
Step-by-step explanation:
Hello,
In this case, the relationship between heat, mass, specific heat and change in temperature is understood by:
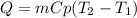
In this case, since the involved substance is water, whose specific heat is 1 cal/(g°C), we compute the heat 4 mL of water need to rise the temperature from 10 °C to 30 °C as shown below:
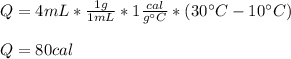
Best regards.