Answer:
-2
Step-by-step explanation:
Hello,
In this case, oxidation numbers represent the degree of oxidation at which an element is. In the most of the cases, the oxidation numbers match with the valence electrons which are outer electrons surrounding an atom. In this particular case, sulfur, which has six valence electrons has an oxidation state of +6 since six electrons are surrounding it at that state, given its electron configuration:
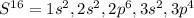
Thus, at level three, we can see 4+2=6 valence electrons, which match with its oxidation state. Nonetheless, it could also have +2, -2 and +4 as its feasible oxidation states, but in this case -2 is the proper oxidation state as it needs 2 more electrons to attain the octet and become an stable chemical species.
Best regards.