Answer:
See explanation.
Step-by-step explanation:
Hello,
In this case, we could have two possible solutions:
A) If you are asking for the molar mass, you should use the atomic mass of each element forming the compound, that is copper, sulfur and four times oxygen, so you can compute it as shown below:
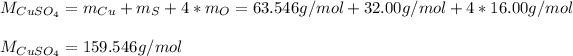
That is the mass of copper (II) sulfate contained in 1 mol of substance.
B) On the other hand, if you need to compute the moles, forming a 1.0-M solution of copper (II) sulfate, you need the volume of the solution in litres as an additional data considering the formula of molarity:
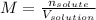
So you can solve for the moles of the solute:
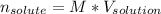
Nonetheless, we do not know the volume of the solution, so the moles of copper (II) sulfate could not be determined. Anyway, for an assumed volume of 1.5 L of solution, we could obtain:
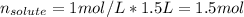
But this is just a supposition.
Regards.