Answer:
THE HALF LIFE OF PHOSPHORUS-32 ISOTOPE IS 14 DAYS.
Step-by-step explanation:
Using the Nerst equation of half life;
t1/2 = t * in(2) / in (No/Nt)
where;
t1/2 = half life = the unknown
t = elapsed time = 56 days
No= initial mass of the sample = 4.0 g
Nt = final mass of the sample = 0.25 g
Substituting the values into the equation, we have;
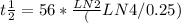 t1/2 = 56 * Ln2 / Ln ( 4/0.25)
t1/2 = 56 * Ln2 / Ln ( 4/0.25)
t1/2 = 56 * 0.6932 / Ln (16)
t1/2 = 56 * 0.6932 / 2.7726
t1/2 = 38.8192 / 2.7726
t1/2 = 14.001 days.
t1/2 = 14 days.
The half life of the phosphorus- 32 would be 14 days