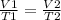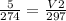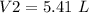Answer:
The volume of balloon is 5.41 L.
Step-by-step explanation:
The volume of helium (V1) = 5 L
Temperature (T1) = 1 degree Celsius
Now covert the temperature into kelvin by simply adding the 273 with given temperature. Thus, 1-degree Celsius = 274 kelvin.
The volume of balloon (V2) =?
Temperature (T2) = 24 degree Celsius
Now covert degree Celsius into kelvin. Thus, 24-degree Celsius = 297 kelvin.
Using Charl’s law.