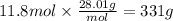Answer:
331 g
Step-by-step explanation:
Step 1: Write the balanced equation
N₂(g) + 3 H₂(g) → 2 NH₃(g)
Step 2: Calculate the moles corresponding to 400 g of ammonia
The molar mass of ammonia is 17.03 g/mol.
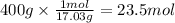
Step 3: Calculate the moles required of nitrogen
The molar ratio of N₂ to NH₃ is 1:2.
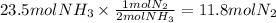
Step 4: Calculate the mass corresponding to 11.8 moles of nitrogen
The molar mass of nitrogen is 28.01 g/mol.