Answer:
The balanced equation is
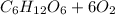 →
→
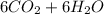
The number of grams of oxygen needed to react with 30 grams of glucose is 30 grams.
Step-by-step explanation:
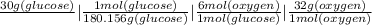
The set-up shown is how this problem can be solve. This is called stoichiometry. Stoichiometry refers to math amongst the elements or the compounds. Now, we solve the equation above.
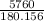
Divide the expression.
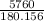 = 31.9722
= 31.9722
Now, we round this answer.
31.9722 → 32
The number of grams of oxygen needed is 32 grams.
Hope this helps!