Answer:
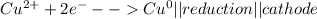
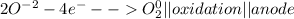
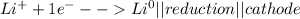
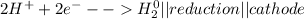
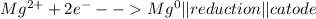
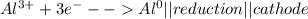
Metal ions have positive charges, and are attracted to the negative electrode (the cathode) and are reduced during electrolysis.
Non-metal ions have negative charges, and are attracted to the positive electrode (the anode) and are oxidized during electrolysis.