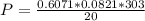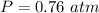Complete Question
The complete question is shown on the first uploaded image
Answer:
The pressure is
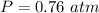
Step-by-step explanation:
From the question we are told that
The mass of the carbon monoxide is
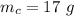
The temperature at which takes place
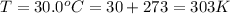
The volume of the sealed vessel is
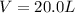
Generally the ideal gas law is mathematically represented as
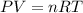
Where R is the gas constant with value
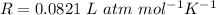
n is the number of moles of carbon monoxide which is mathematically evaluated as
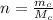
where
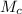 is the molar mass of carbon monoxide which is a constant with value
is the molar mass of carbon monoxide which is a constant with value
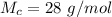
So
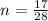
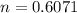
Now Making P the subject we have