Answer: There are
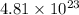 molecules in 0.80 mol of NaCl.
molecules in 0.80 mol of NaCl.
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
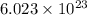 of particles.
of particles.
As 1 mole of
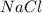 contains =
contains =
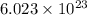 molecules
molecules
0.80 mole of
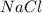 contains =
contains =
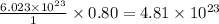 molecules
molecules
Thus there are
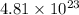 molecules in 0.80 mol of NaCl.
molecules in 0.80 mol of NaCl.