Answer:
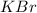 will make an electrolytic solution
will make an electrolytic solution
Step-by-step explanation:
Electrolyte is a substance which dissociates into ions when dissolved in water and thus is able to conduct electric current through them.
Compounds in solid form does not conduct electricity due to the absence of free ions.
a)
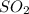 is a covalent compound and thus not an electrolyte.
is a covalent compound and thus not an electrolyte.
b)
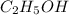 is a covalent compound and thus do not dissolve in water and thus is not an electrolyte.
is a covalent compound and thus do not dissolve in water and thus is not an electrolyte.
c)
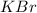 is an ionic compound and thus dissolve in water and give ions which help to conduct electricity.
is an ionic compound and thus dissolve in water and give ions which help to conduct electricity.
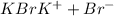
d)
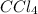 is a covalent compound and thus do not dissolve in water and thus is not an electrolyte.
is a covalent compound and thus do not dissolve in water and thus is not an electrolyte.