Answer:
The answer is "2074.2 KW"
Step-by-step explanation:
The mole part of
 =0.1
=0.1
The mole part of
 = O.19
= O.19
The mole part of
 =0.71
=0.71
At temperature (
 ) mixture receives from turbine =720K
) mixture receives from turbine =720K
At pressure (
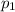 ) mixture receives from turbine =0.35 Mpa
) mixture receives from turbine =0.35 Mpa
Flow rate volumetric V = 3.2
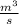
A turbine leaves the blend at temperature (
 ) = 380K
) = 380K
The solution comes out of a pressure (
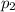 )= 0.11 Mpa
)= 0.11 Mpa
Decreasing healthy mass balance:
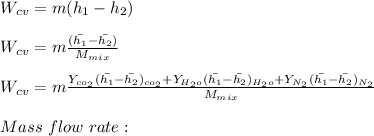
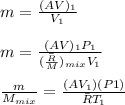
by increasing value we get:
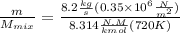
After solve we get = 0.1871
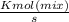
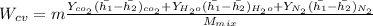
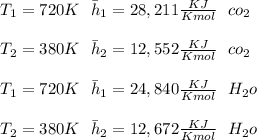
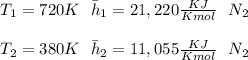
put the value in above given formula it will give
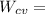 2074.2 KW
2074.2 KW