Answer:
0.713atm for CO and 0.287atm for CO₂
Step-by-step explanation:
Based on the reaction:
FeO(s) + CO(g) ⇋ Fe(s) + CO₂(g)
Kp is defined as:
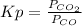 = 0.403
= 0.403
When 1.00 atm of CO react with an excess of FeO, the pressures in equilibrium are:
PCO = 1.00atm - x
PCO₂ = x
Where x represents the reaction coordinate.
Replacing in Kp expression:
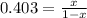
0.403 - 0.403x = x
0.403 = 1.403x
0.287atm = x
Thus, pressures in equilibrium are:
PCO = 1.00atm - x = 0.713atm
PCO₂ = x = 0.287atm