Answer:
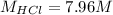
Step-by-step explanation:
Hello,
In this case, since the neutralization reaction between HCl and NaOH is:

We notice a 1:1 molar ratio, for that reason, at the equivalence point we find:
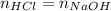
Thus in terms in molarities one could compute the concentration of HCl in the old bottle for the used NaOH for the neutralization as:
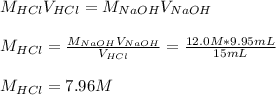
This value is lower than 37% HCl that in molarity is about 12 M, such difference is due to its high volatility.
Best regards.