Answer:
The new pH of the solution
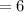
Step-by-step explanation:
Given
pH of bottle of Soda is
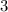
As we know that
pH
 log [H+]
log [H+]
We will derive the hydrogen ion concentration when pH is
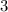
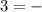 log [H+]
log [H+]
Taking anti-log on both the sides, we get -
H+ ion is equal to
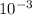
When the hydronuim ion concentration reduces by
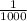 of the original concentration, the new hydrogen ion concentration becomes
of the original concentration, the new hydrogen ion concentration becomes
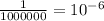
The new pH is
![= - log [ 10^(-6)]\\= 6](https://img.qammunity.org/2021/formulas/chemistry/high-school/t6plujttom6rody4opa0hz4yclnv3vo495.png)