Answer:
All single bonds.
Step-by-step explanation:
To calculate the number of single, double or triple bonds (unsaturations), for molecules containing only carbon, hydrogen, monovalent halogens, nitroge , as the organic molecule C10H22 has, we use the following formula, also known as the hydrogen deficiency index (HDI):
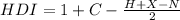
Where
C = number of carbon atoms
H = number of hydrogen atoms
X = number of halogen atoms
N = number of nitrogen atoms
2 gives an equivalent result.
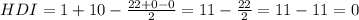
Since the number of unsaturations is zero, it means that the molecule only has single bonds, it does not have double or triple bonds and it does not have rings.