Answer:
Concentration of hydrogen ion is

Step-by-step explanation:
pOH of solution
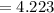
The concentration of OH ions is to be derived first
As we know-
pOH
![= - log [ OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/br7al69kqr9s76q0a1t8t8ivg85hk6tw38.png)
Substituting the given values, we get -
![4.223 = - log [OH^-]\\](https://img.qammunity.org/2021/formulas/chemistry/college/gals267zxcc708wsdolb9zanyopx4qbbdx.png)
Taking antilog on both sides we get -
OH- concentration
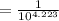
OH- concentration
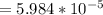
As we know tha
Pkw
![= P[H^+] + P[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/7tksqs6715w5dzx1ooaepiqgbormi0lj90.png)
![14 = P[H^+] + 4.223\\P[H^+] = 14 - 4.223\\P[H^+] = 9.777](https://img.qammunity.org/2021/formulas/chemistry/college/byduyqqpuxlznc2yviknnnadl2y691i1tp.png)
Concentration of hydrogen ion is
![9.777 = - log{H^+]\\log{H^+] = -9.777\\{H^+] = (1)/(10^(-9.777)) \\{H^+] = 1.671 * 10^(-10)](https://img.qammunity.org/2021/formulas/chemistry/college/xrolo2z7pko23e9vtn8v84rxolwtbcqa88.png)
Concentration of hydrogen ion is
